Greetings from Freyr China
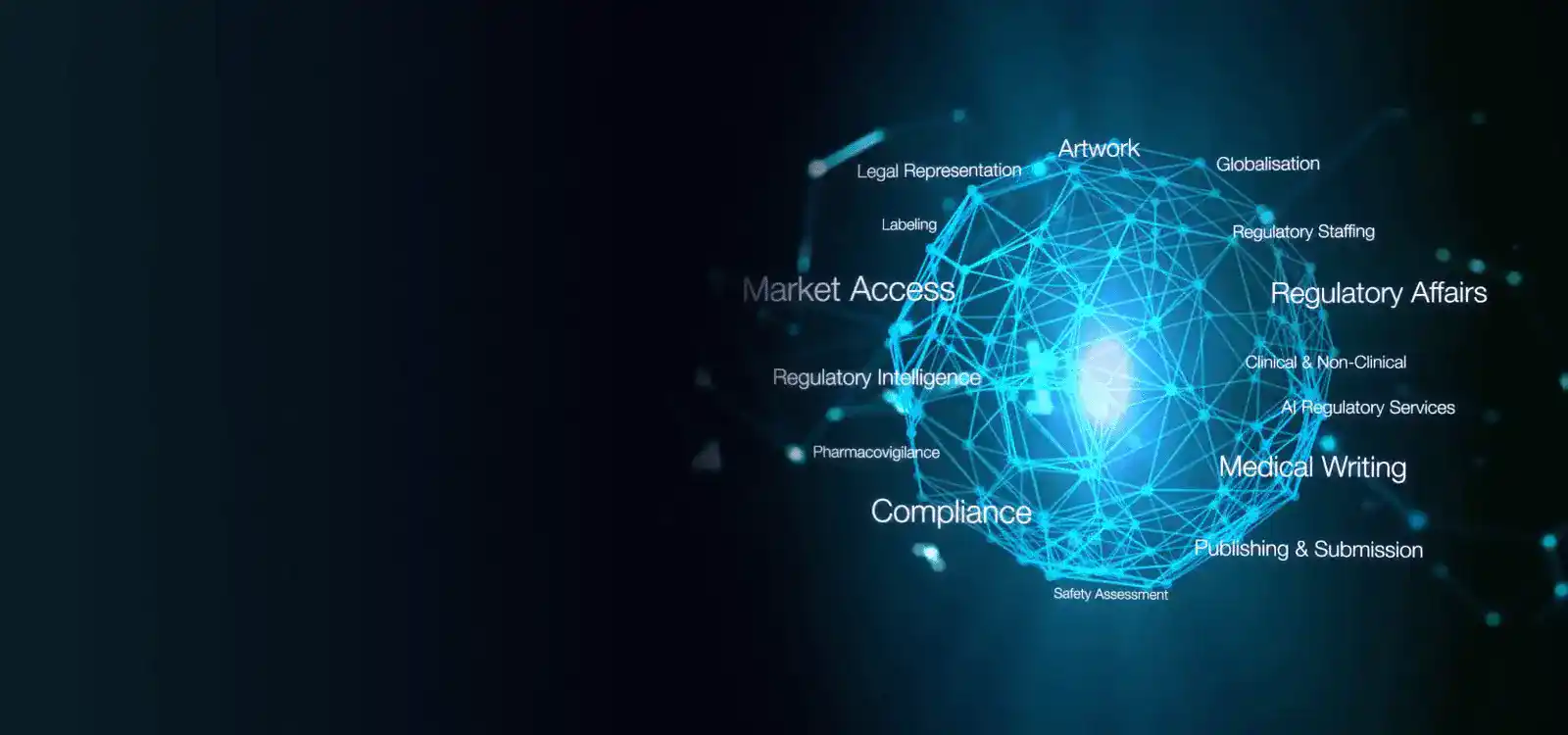
Freyr is a leading Regulatory solutions provider offering comprehensive services to life sciences firms across the regulatory spectrum. With a robust presence spanning over 120 countries, we facilitate the "local to Global" journey for life sciences companies.
In China, Freyr China plays a pivotal role in assisting companies with Drug registration in China under the National Medical Products Administration (NMPA). Our services encompass Regulatory Strategy formulation, Market Intelligence, In-country representation, Post-Market Surveillance (PMS) functions, and more. Additionally, we provide cutting-edge Regulatory software solutions tailored to streamline the entire NMPA registration life cycle covering everything from the China new drug approval process to China generic drug approval process.
Global Presence in
25+ Countries2500+
Inhouse Regulatory
Experts & GrowingSupport in
120+ Countries2100+ Global
clients & growing